Introduction: New theranostic tools for treating and diagnosing disease have driven a sharp increase in demand for new and existing radiopharmaceuticals. Additionally, the development of advanced radiochemical methodologies, like the copper-mediated radiofluorination (CMRF) reaction, has provided unprecedented access to new radiochemical diversity for radiopharmaceutical research. However, bringing new radiochemical methods to bear for addressing complex clinical and preclinical relevant radiosynthetic problems remains a significant challenge. To address these challenges, we aim to explore how data science tools, such as statistical “design of experiments” (DoE) and response surface modeling,1 can be leveraged with the recently developed miniaturized high-throughput experimentation (HTE) workflows2 to rapidly and efficiently solve complex multivariate radiochemical optimization problems and thus drive the preclinical radiopharmaceutical development cycle. To demonstrate this approach, HTE and DoE were used to quickly establish reliable CMRF synthesis conditions for [18F]crizotinib, a difficult-to-access molecule of preclinical relevance whose precursor was only available in limited supply (< 58 µmol).4
Methods: The HTE protocol developed by Webb et al. utilizes commercially available equipment available in standard 24- and 96-well plate format (analytical sales) and can rapidly run and analyze multiple miniaturized radiochemical experiments in parallel.1 Commonly available analytical methods, including RadioTLC, Gamma Counting, and PET, were adapted to rapidly and accurately estimate radiochemical conversion (RCC). JMP discovery statistics software was used to design two experimentally efficient phased DoE studies. A low-resolution categorical variable pilot study (15 experiments) was designed to screen optimal reaction solvent and additive combinations using a model substrate ((2,4-dichloro-3-methylphenyl) boronic acid pinacol ester). These results then informed the design of a response surface modeling study (24 runs) to accurately model the effects of Cu(OTf)2, precursor, ligand, and co-solvent (n-BuOH) loadings on RCC. The 24 reactions were performed in parallel (100 µL solvent) and sealed at 120 °C for 30 min with stirring before reaction analysis via sequential gas scintillation radioTLC. The optimal conditions predicted from the resulting response surface model were validated empirically and automated on a GE tracer-lab module.
Results: The pilot categorical variable screening experiments identified imidazo[1,2-b]pyridazine (IMPY) and DMI as the optimal copper ligand and solvent for labeling the model precursor. The high-resolution 24-run, 4-factor D-optimal response surface modeling study then identified the aryl boronate pinacol ester crizotinib precursor (1.8 µmol) and IMPY (37 µmol) as having significant effects on reaction performance. The amount of Cu(OTf)2 (set 2.7 µmol) and %n-BuOH (set 0% vol)co-solvent were not found to affect reaction performance significantly. Full-scale validation experiments using the optimal conditions predicted by the resulting response surface model afforded [18F]crizotinib with a 57% RCC (n =1; predicted 55% RCC). An alternative set of suboptimal conditions requiring less than half the expensive precursor was also tested and afforded the product with an acceptable 40% RCC (n = 1, predicted 36% RCC). These conditions were validated for automated production using a TRACERlab FX2 N (GE) synthesis module (n =1; AY ca 17%).
Conclusion: This study demonstrates how data-driven approaches like DoE can be combined with innovative high-throughput experimentation workflows to systematize and accelerate radiochemical process optimization. The miniaturized HTE protocol allowed the entire combined 39-run DoE variable screening and response surface optimization experimental regime to be carried out in two 3-hour experimental sessions using just 27.8 µmol of the valuable and limited precursor, quickly affording adequate and automatable radiolabeling conditions for the production [18F]crizotinib. The use of these methods thus has the potential to condense the overall radiopharmaceutical development timeline from months to weeks or days. As the demand for new radiopharmaceuticals continues to grow, data-driven approaches that facilitate efficient radiopharmaceutical production and rapid radiochemical research throughput will be crucial to ensuring a steady supply of novel and clinically impactful radiopharmaceuticals.
Image/Figure: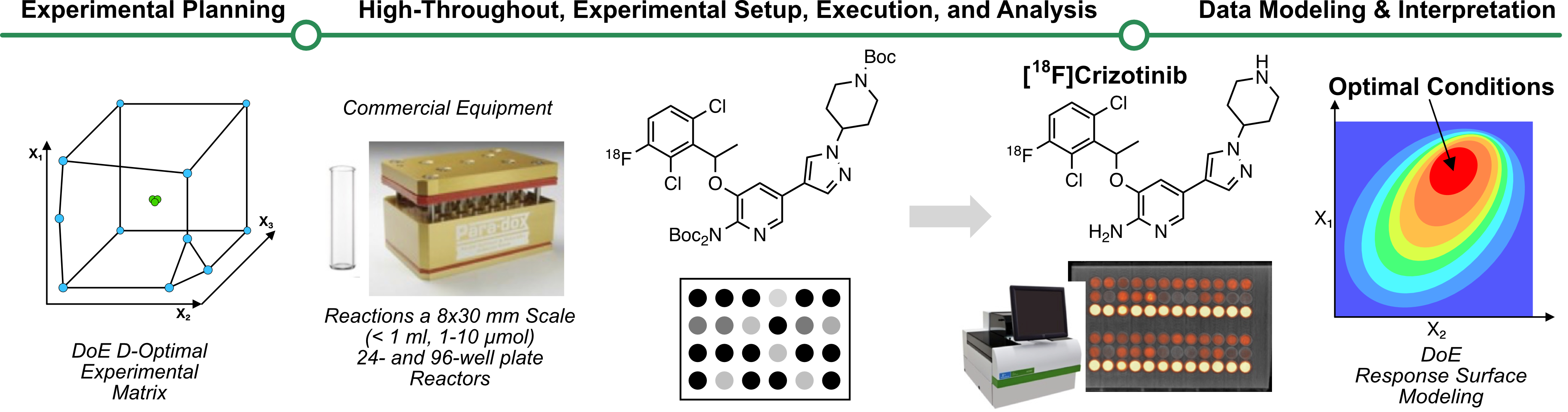
Click to view full size
Image/Figure Caption:
Statistically optimal “design of experiments” (DoE) can be leveraged with high-throughput radiochemistry to develop detailed response surface models to guide decision-making and expedite the radiopharmaceutical development cycle.
Author
University of Michigan Medical School