Magnetic Particle Imaging (MPI) is a novel imaging technique known for its high spatial resolution and absence of background noise.[1] It produces images of superparamagnetic nanoparticles by exploiting the non-linear magnetization response of these particles to an alternating magnetic field. The MPI signal can penetrate tissues without attenuation due to the low excitation frequencies, enabling noninvasive measurements within deep organs. Importantly, magnetic nanoparticles are considered safe for clinical research, with iron oxide already approved by the FDA for clinical trials.[2] MPI has the potential to offer sensitive, real-time monitoring of various biological activities in vivo and could be translated for clinical imaging. However, unlike activatable fluorescent or MRI probes, there have not been activatable MPI tracers that can function to sense the activity of a biological target (e.g., an enzyme).
Distance-Dependent Magnetic Resonance Tuning (MRET) is an imaging technology based on Magnetic Resonance Imaging (MRI) that can quantitatively detect biological activities involving physical distance changes.[3] The MRET contrast agent is composed of a superparamagnetic core and a cleavable paramagnetic metal chelate shell. The T1 relaxivities of the paramagnets and the T2 relaxivities of the superparamagnets can be simultaneously switched on and off based on their distances due to their magnetic interactions.[4] In this study, we explored a similar approach to designing activatable MPI probes for in vivo molecular imaging and activity sensing.
Here, for the first time, we demonstrated a new nanoprobe as an MPI agent featuring activatable contrasts. The nanoprobe consists of the superparamagnetic iron oxide nanocrystal cluster and the paramagnetic MPI quencher (Figure 1a). The magnetic cluster exhibits superior magnetic properties and has excellent intrinsic MPI contrast with a limit of detection of 10 ng Fe. The signal per mass iron of the magnetic cluster is more than twice that of commercially available Vivotrax, the gold standard for MPI contrast agents. As the quencher is chemically conjugated to the surface of the iron oxide, due to magnetic interactions, the MPI signal of the nanoprobe is reduced by 70% (Turn-off). When the quencher is removed from the surface, the MPI signal can be effectively recovered to the original state (Turn-on). Based on the reversible quenching property, bio-cleavable linkers such as a disulfide bond and protease substrate peptide have been used to develop activatable MPI probes. It was demonstrated, both in vitro and in vivo, that with the reducing agent (GSH) and the protease excreted in the liver, the quencher is rapidly cleaved from the iron oxide surfaces, leading to a 100% increase in the MPI signals (Figure 1b and c). With tunable contrast and excellent sensitivity, this strategy of reversible MPI quenching can offer new opportunities for molecular diagnostics and various image-guided biomedical applications.
Image/Figure: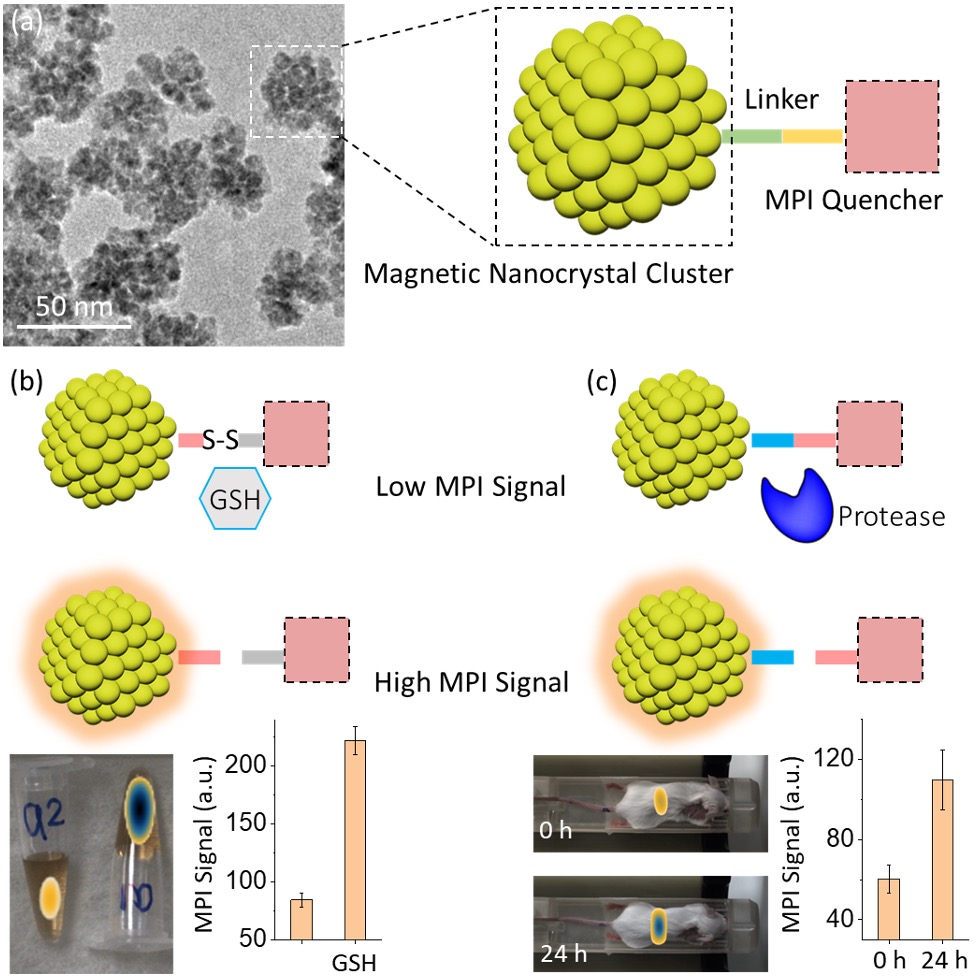
Click to view full size
Image/Figure Caption:
Figure 1. Design of activatable MPI nanoprobes for MPI sensing of biomolecular activity. (a) The nanoprobe consists of magnetic nanocrystal clusters and a chemically conjugated quencher using target-cleavable linkers. When subjected to an external field, the quencher interacts with the superparamagnetic core, resulting in a reduced MPI signal. The MPI signal can be subsequently activated by removing the quencher. (b-c) Activity-sensing MPI probes were prepared using the disulfide linker (b) and protease substrate peptide linker (c), in which the MPI signal can be turned on by the addition of GSH and protease in the liver, respectively.
Author
Stanford University