Abstract
Background: Tumor-associated angiogenesis plays a critical role in therapeutic targeting and detecting, serving as a key marker for tumor development and response to treatment. Unlike normal vasculature, which is characterized by its organized structure, controlled permeability, and efficient blood flow, tumor vasculature exhibits abnormal phenomena, characterized by a disorganized architecture, accompanied by increased permeability that leads to pronounced leakage and a markedly reduced blood flow. In vitro angiogenesis assays and Artificial Intelligence (AI)-driven analysis have gained popularity for their ability to model tumor angiogenesis, there remains a significant gap in the utilization of in vivo imaging techniques for the accurate classification of vasculature within cancer models. Here, we propose a novel automatic classification technique for specific angiogenesis via AI using fluorescent-Dextran based intravital imaging in squamous cell carcinoma of head and neck cancer (SNHCC) models to identified normal, malignant, and cancer models with different treatment responses. Focus was given to accurate differentiation of therapeutic efficacy between Cetuximab, a clinically popular targeted therapy for SNHCC and combination therapy of Cetuximab with Magrolimab, an emerged innate immune checkpoint immunotherapy.
Method: We developed an automatic vasculature classification technique utilizing a multi-level ensemble method. This approach integrates predictions from both Convolutional Neural Networks (CNNs: DenseNet121, Vgg16, ResNet50) and traditional machine learning classifiers (MLs: SVM, KNN, Naive Bayes) to not only distinguish between normal and malignant tumor models but also to assess the varied therapeutic outcomes of Cetuximab treatment alone and its combination with Magrolimab for SNHCC. To acquire vasculature images, intravital fluorescent imaging was conducted on murine xenograft models of SNHCC (FaDu cell injected model) both treated and untreated, together with normal mice, following the intravenous injection of Dextran-Tetramethylrhodamine (TRITC). A dataset comprising 784 in-vivo images and 471 quantitative vascular records was selected for the training and validation of our models. The ensemble method was refined with the introduction of a novel Integrated Curvature Estimation (ICE) approach, innovatively designed to approximate the curvature of vasculature, a parameter not obtainable through traditional quantitative tools. Additionally, we incorporated an adaptive weight board learning system to update weights dynamically, in order to address the challenge of data imbalance. Predictions from CNNs and MLs were first averaged and then unified through Bootstrap Aggregating.
Results: The ensemble model utilizing intravital images and vascular records together demonstrated superior performance, achieving 96.25% accuracy (ACC), 0.99 area under the curve (AUC), 96.06% sensitivity (SEN), and 98.73% specificity (SPE). In contrast, the image-based model presented only 93.75% ACC, 0.98 AUC, 93.70% SEN, and 97.88% SPE and the vascular record-based model showed the worst performance with ACC of 75.00%, AUC of 0.93, SEN of 71.25%, and SPE of 91.39%.
Conclusion: The presented ability of AI-driven differentiation of angiogenesis together with intravital imaging technique provides a key clinical parameter for tumor staging and an effective indicator for preclinical evaluation of drug efficacy. Such strategy is potentially valuable for the quick and reliable evaluation of new drugs and the treatment planning of clinical trials, particularly with the growing interest in combination and synergistic immunotherapies and targeted treatments.
Keywords: Angiogenesis, Ensemble learning, Squamous cell carcinoma of head and neck cancer, Cetuximab, Magrolimab, Therapeutic outcome prediction
Image/Figure: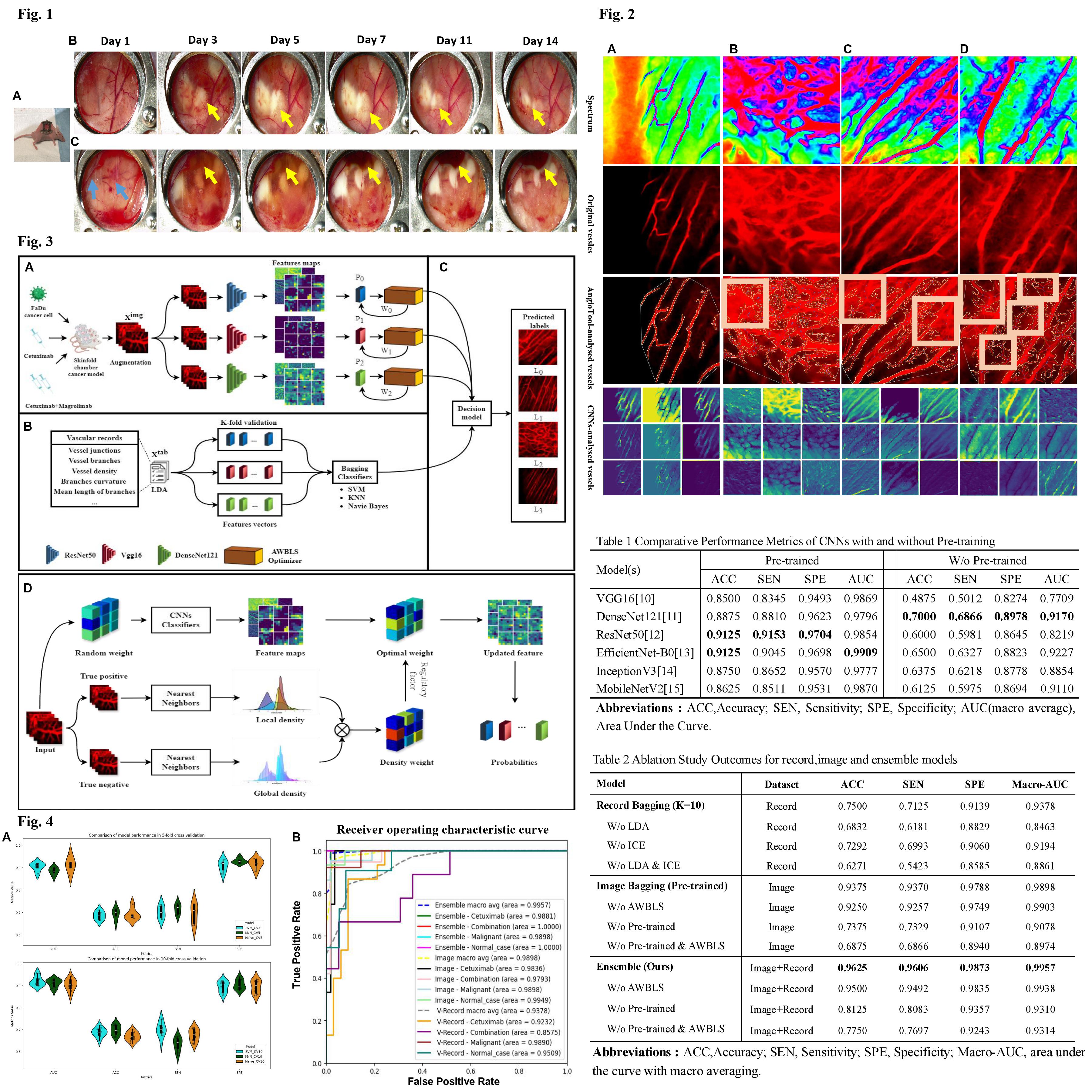
Click to view full size
Image/Figure Caption:
Figure 1: Fluorescent dextran-based tumor vasculature imaging in skinfold cancer models under different treatment regimens (A) Intravital imaging based on skinfold cancer models(B) Representative time-lapse anatomical imaging of tumor growth after combinational cetuximab-targeted therapy and anti-CD47 immunotherapy (C) Representative time-lapse anatomical imaging of Tumor-associated vascular obstruction phenomenon. As the first step towards the implantation of a skinfold chamber, 8-10 weeks old male nude mice underwent the surgical insertion of a dorsal skin window chamber, which provided a favorable basis for conducting intravital imaging (Fig.1A). The anatomical imaging depicted the generation of tumor nodules and the fine details of angiogenesis in skinfold models. Following the combinational cetuximab-targeted therapy and anti-CD47 immunotherapy, the size of the tumor nodules was dramatically reduced and even disappeared, as showed by yellow arrow (Fig.1B). As tumors grow in size, the interstitial fluid pressure may increase to the extent that small blood vessels are consistently blocked. This results in a stagnation of blood flow and the development of necrotic regions, usually found close to the tumor area (Fig.1C).
Figure 2: Comparative vascular analysis across treatment responses in tumor models. Row ‘Spectrum’ employs spectral imaging to highlight vascular heterogeneity. ‘Original Vessels’ row captures the intrinsic fluorescent imaging of the vasculature. ‘AngioTool-Analysed Vessels’ illustrates the vasculature post-analysis by the AngioTool software, emphasizing vessel morphology and density. ‘CNNs-Analysed Vessels’ showcases the application of convolutional neural networks in extracting and analyzing vascular features. Columns (A) through (D) correspond to: (A) Normal cases (B) Malignant models show an abnormal, leaky vasculature network with new capillary formation due to its rapid tumor growth and metastasis. (C) Cetuximab-treated models. (D) Combinational-treated (Cetuximab + Magrolimab) models. This figure also reveals the shortcomings inherent in conventional quantitative tools and Convolutional Neural Networks (CNNs) for vascular analysis. It highlights instances where traditional analyses, such as those conducted by AngioTool, struggle with accurate segmentation of actual vascular areas — a limitation vividly depicted in the misidentified regions within white boxes(as shown in Fig 2-B, C, D).
Figure 3: Overview of the training strategy (A) Training method based on imaging data. Intravital fluorescent imaging were performed on murine xenograft models with different treatment plans. CNNs with AWBLS optimizer were trained to discriminate In-vivo images under various treatment responses. (B) Training method based on vascular records data. Linear discriminant analysis and K-fold (K=5,10) validation were applied to decrease the dimension and improve the model’s generalization, respectively. (C) Decision model with a unique voting scheme addresses data imbalance across modalities. Abbreviations: SVM: Support Vector Machine, KNN: K-Nearest Neighbors, LDA: Linear Discriminant Analysis, Bagging: Bootstrap Aggregating, AWBLS: Adaptive Weighted Broad Learning System. (D) An overview of the application of the Adaptive Weighted Broad Learning System (AWBLS) in our study. Initially, we classify the input images into true positives, where the label matches the image, and true negatives, which comprise the remaining images. We then use the nearest neighbor method to extract their local and global densities. By mixing these densities with a trade-off parameter, we can obtain a density weight which allows our model to adjust the weight of less numerous samples, paying more attention on samples that are hard to distinguish.
Figure 4: Metrics performance visualization (A) Violin box plot comparison of SVM, KNN, and Naive Models based on vascular records dataset in 5-fold and 10-fold cross-validation (B)Receiver operating characteristic curve of each method Table 1 Comparative Performance Metrics of CNNs with and without Pre-training
Table 1: Comparative Performance Metrics of CNNs with and without Pre-training presents a striking contrast in performance metrics between pre-trained and non-pre-trained CNNs applied to a vascular classification task. The results are particularly illuminating when considering the substantial enhancements pre-training confers across all models.
Table 2: Ablation Study Outcomes for record,image and ensemble models. Table 2provides a summary of the performance comparisons from our ablation studies, the ensemble model outperformed the individual image and record bagging models. For the record data Bagging approach, we streamlined the computational process by calculating the average values of each metric obtained from 10-fold cross-validation in advance. In our ablation trials, we eliminated LDA and ICE, respectively. In the image Bagging (Pre-trained) experiments, we conducted an ablation study on AWBLS.
Author
student
The Hong Kong Polytechnic University