Background: Cellular senescence is a permanent state of cell cycle arrest characterized by increased activity of senescence associated β-galactosidase (SA-β-gal)1,2. Notably, cancer cells can also enter a senescent state induced by anticancer therapies and be considered for sequential treatment with pro-senescence followed by senolysis3,4. However, there is currently no effective imaging agent targeting β-galactosidase (β-Gal) for imaging and monitoring senescent cancer cells in vivo. Aggregation-induced emission luminogen (AIEgen) demonstrates strong fluorescence, good photostability, and biocompatibility, making it a potential candidate for imaging and monitoring senescent cancer cells in vivo when endowed with β-Gal-responsive capabilities5,6.
Objective: It aims to report a β-Gal-activated AIEgen called QM-β-gal for cancer senescence imaging and senolysis monitoring in vivo.
Methods: QM-β-gal was synthesized and characterized. The absorption/emission spectra, specific enzyme responses, and size changes were analyzed before and after activation by β-Gal. Doxorubicin (DOX) was used to establish senescent cancer cell models. Cells incubated with QM-β-gal were imaged and β-Gal targeting capability was evaluated by specific small interfere RNA (siRNA) inhibition and colocalization with organelle dyes. The superior of QM-β-gal was assessed by comparison with commercial β-Gal fluorescence detection. And the biocompatibility was evaluated by CCK-8 assay. For in vivo investigations, tumor-bearing mouse model was induced into cancer senescence by DOX and injected with QM-β-gal through tail vein to image senescent cancer cells in vivo. Mice were sacrificed to collect serum, tumor, liver and kidney at various time points to quantify circulation half-time and metabolism of QM-β-gal. Blood serum biochemical tests and major organs HE staining were implemented to evaluate biosafety in vivo. ABT263 was used as senolytics on DOX-induced senescent cancer cells and mice with senescent tumor. To monitor the senolysis, QM-β-gal was injected to monitor the process of senolysis induced by ABT263 on DOX-induced senescent tumor in vivo.
Results: QM-β-gal exhibited good amphiphilic properties and formed aggregates that emitted fluorescence upon β-Gal activation. The absorbance had two peaks at 358 nm and 445 nm, while nearly no emission and fluorescence in solvents. The enhanced fluorescence of QM-β-gal was only observed in incubation with β-Gal compared to other compounds, suggesting its good specificity to β-Gal. An emission peak at 558 nm of QM-β-gal appeared and noticeable nanoaggregates were formed after β-Gal activation. For in vitro studies, MDA-MB-231 and HCT116 showed typical cellular senescence phenotypes induced by DOX, including higher SA-β-gal and increased β-Gal expressions. QM-β-gal showed high specificity towards the increased activity of β-Gal in lysosomes and imaged DOX-induced senescent cancer cells with no cytotoxicity. QM-β-gal also showed higher fluorescence intensity, better photostability, and more sensitive response in vitro than commercial detection. For in vivo investigations, DOX also induced tumor senescence. QM-β-gal exhibited rapid metabolism in vivo with circulation half-time as 0.2~0.3 h, while accumulated and activated in senescent tumor and mainly cleared and excreted through kidney. QM-β-gal could image senescent cancer cells in vivo for over 14 days with excellent biocompatibility. Moreover, QM-β-gal could allow for monitoring DOX-induced senescent cancer cell clearance in vitro and in vivo during ABT263-induced senolysis.
Conclusions: We reported an enzyme-activated AIEgen (QM-β-gal) to target the increased expression and activity of β-Gal in lysosomes of senescent cancer cells. QM-β-gal could image and monitor senescent cancer cells during pro-senescence therapy with DOX followed by senolysis with ABT263 both in vitro and in vivo. It exhibited rapid clearance but stable retention over time after activation within DOX-induced senescent tumors. Notably, QM-β-gal demonstrated long-term imaging capabilities for senescent cancer cells lasting over 14 days throughout the sequential treatment process. It had the potential to be a promising candidate for labeling senescent cells with high specificity and stability for fluorescence imaging in vivo.
Image/Figure: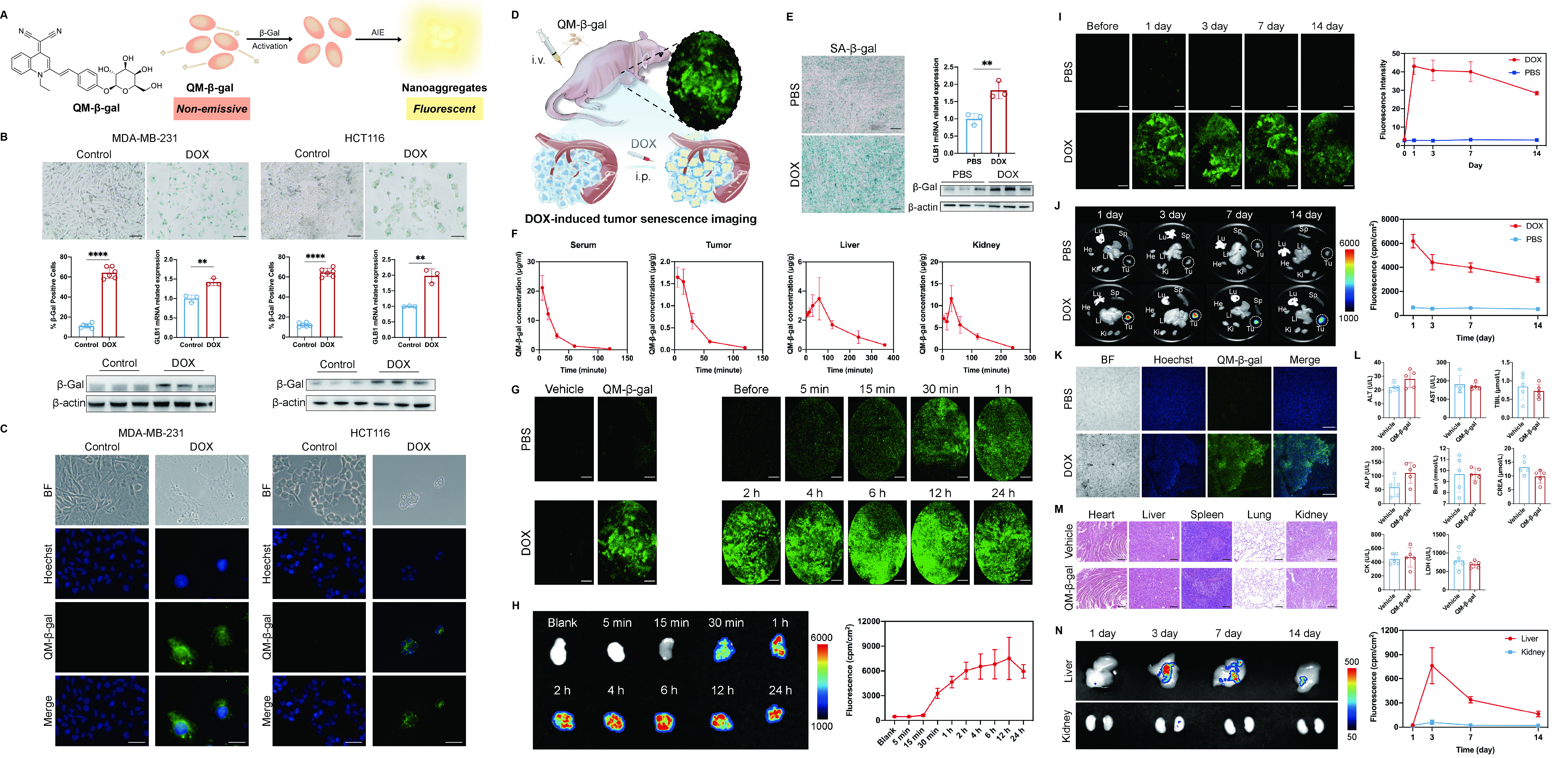
Click to view full size
Image/Figure Caption:
A) Chemical structure of QM-β-gal and illustrated process of activation by β-Gal; B) SA-β-gal staining, percentage of β-Gal positive cell number, GLB1 mRNA expression and β-Gal expression in DOX-treated MDA-MB-231 and HCT116 compared with the controls (t test, scale bar = 100 μm); C) Fluorescence imaging by QM-β-gal with nuclei staining by Hoechst in senescent MDA-MB-231 and HCT116 cancer cells compared to the controls (scale bar = 20 μm); D) Illustration of DOX-induced cancer senescence and fluorescence imaging by injection of QM-β-gal through tail vein on MDA-MB-231 tumor-bearing nude mice; E) SA-β-gal staining of tumor slices, and GLB1 mRNA and β-Gal expression of tumor tissues between PBS and DOX groups (scale bar = 200 μm); F) The concentrations of QM-β-gal at different time points in the serum, tumor, liver, and spleen of DOX group (n = 3); G) In vivo optical fiber confocal imaging in the tumors of PBS and DOX groups with/without QM-β-gal injection, as well as imaging at different time points in the tumor of DOX group with QM-β-gal injection (scale bar = 100 μm); H) Ex vivo fluorescence imaging and quantitative analysis at different time points on tumors of DOX group (n = 3); I) In vivo optical fiber confocal fluorescence imaging and fluorescence intensity of tumors in PBS and DOX groups before and after QM-β-gal injection (n = 4 in both groups, scale bar = 100 μm); J) Ex vivo fluorescence imaging of tumors and major organs containing heart, lung, liver, spleen, and kidney at 1, 3, 7, and 14 days after QM-β-gal injection in PBS and DOX groups (He: heart, Lu: lung, Li: Liver, Sp: spleen, Ki: Kidney, Tu: tumor), as well as fluorescence intensity of tumors in PBS and DOX groups before and after QM-β-gal injection (n = 4 in both groups); K) Confocal fluorescence imaging of ex vivo tumor slices with QM-β-gal and Hoechst in PBS and DOX groups after 14 days post injection (scale bar = 250 μm); L) No significance in serum biochemical indexes containing ALT, AST, TBiL, ALP, Bun, CREA, CK, and LDH (n = 5 in both groups); M) HE staining of major organs including heart, liver, spleen, lung, and kidney, after 14 days post QM-β-gal or vehicle injection on tumor-bearing mice (scale bar = 250 μm); N) Ex vivo fluorescence imaging and quantitative analysis of liver and kidney at 1, 3, 7, and 14 day after QM-β-gal injection in DOX group (n = 3). (**: p<0.01, ****: p<0.0001)
Presentation Poster:
Click to View
Author
Zhejiang University