Cellular senescence was originally categorized as an intrinsic process associated with aging and age-related diseases. Recent studies have shown that common anticancer therapies, such as chemotherapy and radiation therapy, can also push cancer cells into a senescent state termed therapy-induced senescence (TIS). Persistent TIS cells promote tumor proliferation and metastasis. Hence, the objective of our study is to develop a nanoprobe to detect and eventually eliminate residual TIS cells. Here, we have developed a novel near-infrared fluorogenic nanoprobe, named D3, which can only be activated by highly elevated levels of reactive oxygen species (ROS), critical players in the induction and maintenance of senescence. To induce TIS, MDA-MB231 cells were treated with palbociclib, a CDK4/6 inhibitor. Significant growth arrest was observed in senescent MDA-MB231 (SN_MB231) cells which showed 7-fold decreased confluency compared to the untreated MDA-MB231 control cells. Additionally, various senescence markers, including senescence-associated beta-galactosidase (SA-β-Gal), p21, and γH2AX, increased in SN_MB231 cells. The strong signals of mtSOX (for mitochondrial superoxide) and dichlorofluorescein diacetate (DCF-DA) (for a broad spectrum of ROS) indicated remarkably high ROS levels in the senescent cells. For D3 imaging, both MDA-MB231 and SN_MB231 cells were incubated with D3 and examined for cy5.5 fluorescence signal by fluorescence microscope. The cy5.5 signal was only seen in the cytoplasm of SN_MB231 cells, but not in the control MDA-MB231 cells whose ROS levels was too low to activate the D3 signal. Upon systemic injection into the palbociclib treated SN_MB231 tumor-bearing mice, the D3 nanoprobe was specifically turned on by senescence-associated ROS in the senescent tumors. The fluorescence signal at senescent tumors was 3-fold higher than that of non-senescent tumors. In addition, we repeated the same experiment with 4T1 murine mammary tumor models in Balb/C mice to confirm that TIS can be induced by pabociclib and detected by D3 in this immunocompetent syngeneic mouse model, which was confirmed by bioluminescence imaging and cryo-fluorescence tomography. Dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) scans were also conducted to non-invasively measure tumor morphological and microenvironmental changes induced by palbociclib treatment. The tumors of treated mice tend to have heterogeneous enhancement and irregular shapes, as opposed to more round shapes of control tumors. The treated mice also showed increased interstitial space volume fraction. Importantly, we confirmed that the fluorescence intensity of D3 was dependent on the ROS production level which corresponds to senescence progression. D3 activation was also checked in aging normal cells. The ROS level of 12-month-old fibroblasts was 2.3-fold higher than that of embryonic fibroblasts and D3 signal correlated well with the ages of the tested fibroblasts. This results indicate that D3 can be a useful tool to study normal aging process. This groundbreaking design of D3 introduces a novel activation mechanism and a powerful imaging nanoprobe to identify and assess cellular senescence in living organisms.
Image/Figure: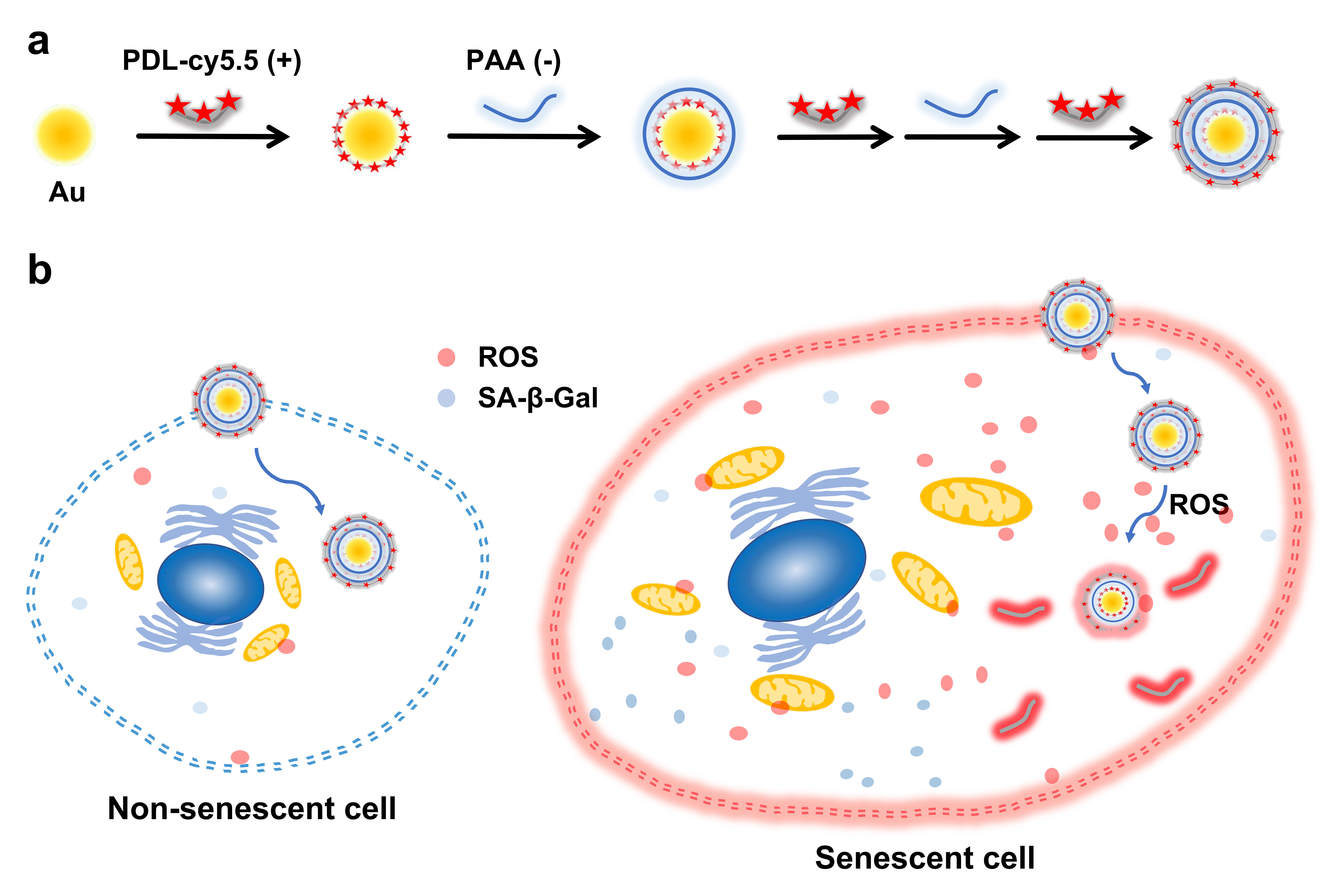
Click to view full size
Image/Figure Caption:
Preparation of ROS responsive nanoprobe, D3. (a) Schematic illustration of D3 preparation by electrostatic interaction. (b) The diagram illustrates the cellular senescence imaging using D3 nanoprobe targeting the senescence-associated ROS in the enlarged senescent cells.
Author
Assistant Professor of Cell Biology Research
Weill Cornell Medicine