Objectives: The development of novel efficient targeted and immuno-therapies requires our improved understanding of the molecular expression profile across patient’s tumor, including personalized molecular profiles for each patient, in order to achieve a better therapeutic response. Raman imaging emerges as a promising avenue, leveraging nanoparticle (NP)-based contrast agents with narrow spectral features and high molecular specificity.1 The high sensitivity of surface-enhanced Raman spectroscopy (SERS) NPs elevates Raman scattering intensities to compete with fluorescence, while offering unparalleled multiplexing capabilities. In this work, we introduce a comprehensive library comprising of 26 distinct SERS NPs, each with high spectral uniqueness, achieved by in silico calcualtions2 and assessment of spectral orthogonality.3 Importantly, we study the quantitative accuracy of the multiplexed imaging across both resonant (SERRS)4 and non-resonant (SERS) NP types. These NPs have the potential to empower researchers and clinicians by enabling the unmixing of up to 26 components within a single imaging pixel. Our study demonstrates the efficacy of these SERS NPs in immune profiling human tissue sections, facilitating highly multiplexed optical imaging. This research underscores the expansive potential of SERS-based Raman imaging in highly multiplexed molecular profiling, promising advancements in personalized medicine, and improved patient outcomes.4
Methods: Fabrication of NPs: (1) creation of 60-nm spheric AuNPs; (2) labeling of AuNPs with unique Raman reporter; (3) silica coating; (4) thiolation; (5) conjugation with biotargeting species; (6) capping remaining thiols; (7) purification.SERS spectra simulation: Density functional theory (DFT) facilitated selection of Raman reporters which met the key criterion of high spectral orthogonality (persuasive data, Fig. S1).2,3 Multiplexed imaging: We assessed the multiplexing capabilities of the imaging nano-probes using 2.5 mW laser power and 0.1 s acquisition time for non-resonant Raman reporters: BPE, 44DP, PTT, PODT, and BMMBP and resonant with 785 nm laser wavelength conventional heptamethine cyanine NIR dyes: IR-775, DTTC, IR-780, IR-792, and IR-797.5 Human tissue imaging: Molecular profiling was performed on 4-μm thick human tonsil tissue sections (N = 10). Human tonsil tissue sections were stained with SERS NPs for CD3, CD20, and isotype control IgG. Each specimen was stained with 250 μL of 50 pM SERS NP staining solution for 20 min.
Results: Herein, we present an expanded library of 26 individual batches of SERS NPs, each with their own unique spectral fingerprint (Fig. 1B). When mixed in a single well, all 26 SERS NPs demonstrated successful spectral unmixing and identification from a single Raman imaging acquisition (Fig. 1C). Although the resonant NPs demonstrated higher absolute Raman intensities, the NIR-resonant Raman reporters exhibited a higher background when excited at 785 nm and more crowded Raman spectra due to their bulkier molecular structures (Fig. 1F). This decreased their ability to be accurately detected in their mixtures. Active biomarker targeting was assessed with Raman imaging on human FFPE tonsil tissue sections by conjugating SERS NPs with CD3 (231±32 antibodies per NP) and CD20 antibodies (256±39 antibodies per NP), respectively. CD3- and CD20- Raman imaging channels showed excellent spatial co-registration with the IHC stains (Fig. 1G). Additionally, we observed strong spatial correlation of T cell distribution outside follicular zones (r = 0.764) and B cell distribution inside follicles (r = 0.936).
Conclusion: Our study revealed the superior multiplexing potential of non-resonant SERS NPs over resonant NPs. Crowded spectra observed with resonant NPs can ultimately lead to higher false-positive results in a patient sample after the spectral deconvolution. Thus, we showcased that these factors are important to consider when developing new nano-based Raman imaging strategies for various multiplexing biomedical applications. In conclusion, our ability to deconvolve 26 SERS NPs in a single imaging pixel offers researchers and clinicians crucial quantitative molecular insights to improve patient care and outcome.
Image/Figure: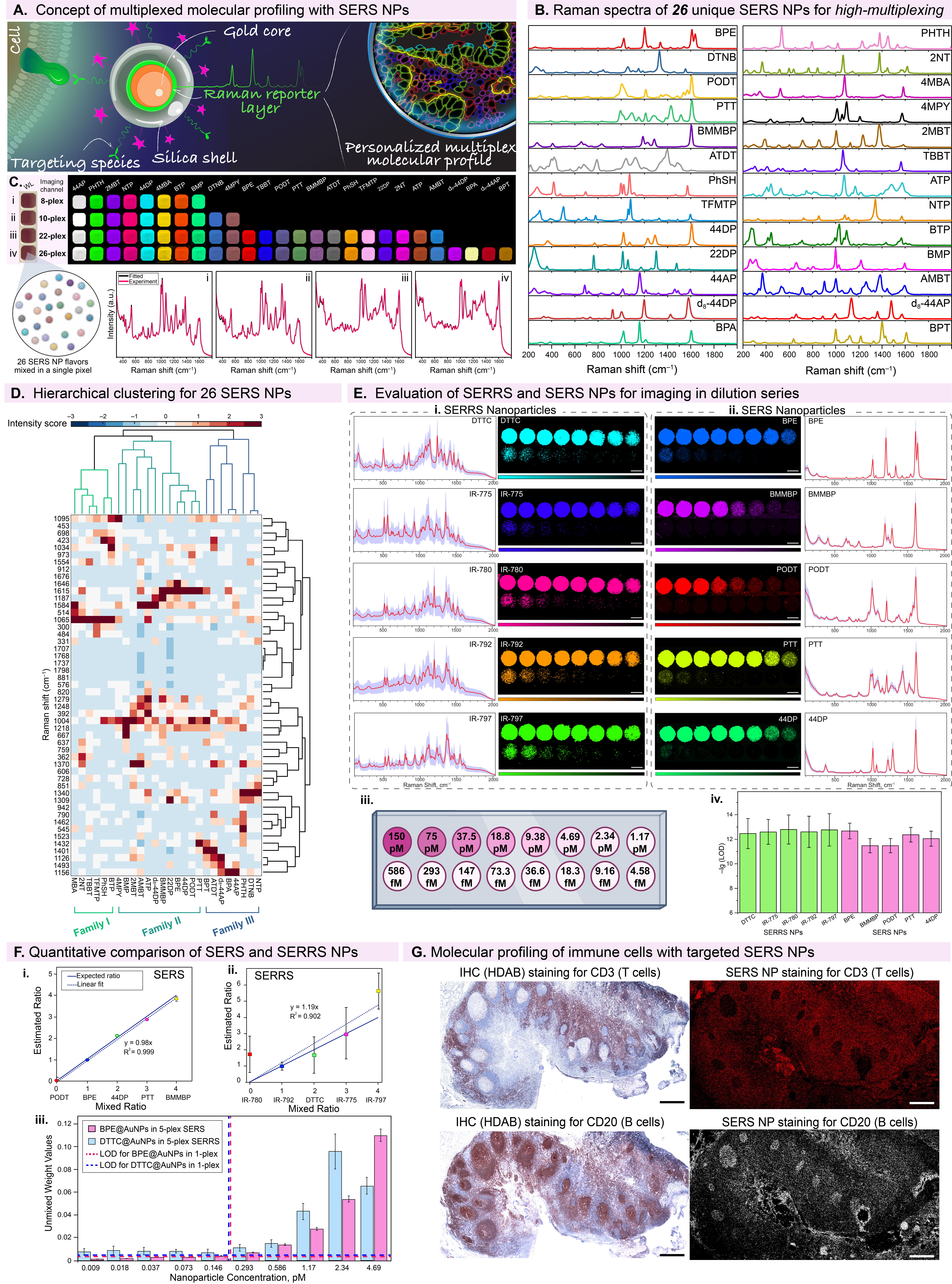
Click to view full size
Image/Figure Caption:
Figure 1. Multiplexing capabilities of Raman imaging in conjunction with SERS NPs to enable highly specific molecular expression and spatial profiling. (A) Architecture of SERS NPs consisting of a 60-nm gold core labeled with one of 26 Raman reporters and coated with a silica shell. Each SERS NP flavor emits the desirable unique spectral signature with narrow Raman bands, suitable for high degree of multiplexing. (B) Spectral library of 26 SERS NPs emitting unique spectra. (C) Increasingly high-plex mixtures mapped colocalized in a single well and hierarchical clustering of 26-plex SERS library features. White light (WL) image and SERS imaging channels of the demultiplexed Raman image depicting the mixed spectrum’s content of the given SERS NP’s signature. For demonstrating multiplexed analysis, each imaging channel was artificially assigned a different color from the 26-color palette. (D) Hierarchical clustering dendrogram of the library of 26 SERS NPs. The clustering was performed on baseline-subtracted SERS spectra normalized to unit variance. For this grid, hierarchical clustering has arranged the SERS flavors into three vibronic Families labeled I, II, and III. Spectral intensities along the horizontal axis have been arranged to highlight the spectral features that are shared among that vibronic family and indicate how that family differs from the others. (E) Sensitivity comparison of SERRS versus SERS NPs in Raman imaging experiments. The average Raman spectra with standard deviation (shaded area, n = 500) for (i) 37.5 pM SERRS NPs (labeled with DTTC, IR-775, IR-780, IR-792, or IR-797) and (ii) 37.5 pM SERS NPs (labeled with BPE, BMMBP, PODT, PTT, or 44DP). Raman imaging channels for the dilution series of SERRS NPs (i) and SERS NPs (ii) starting from 150 pM with 2× dilution steps. Scale bars represent 5 mm. Scheme of nanoparticle concentrations applied onto the coffee filter paper substrate (iii). Limits of detection (LODs) for SERS and SERRS NPs (iv). (F) Spectral unmixing accuracy. The estimated ratios of the five-color SERS (i) and SERRS NPs (ii) from the spectral unmixing, which were normalized to the average concentration of the BPE-labeled and DTTC NPs, respectively. The SERS NPs were mixed with a 0 : 1 : 2 : 3 : 4 ratio of PODT, BPE, 44DP, PTT, and BMMBP-labeled NPs, respectively. The SERRS NPs were mixed with a 0 : 1 : 2 : 3 : 4 ratio of IR-780, IR-792, DTTC, IR-775, and IR797-labeled NPs, respectively. (iii) Unmixed weight values for BPE-labeled NPs in the presence of 37.5 pM BMMBP, PODT, PTT, and 44DP- labeled NPs and DTTC-labeled NPs in the presence of 37.5 pM IR-775, IR-792, IR-780, and IR-797-labeled NPs, along with the LOD concentration for each NP flavor in 1-plex, which can be used as a ground rule for assessing the impact of multiplexing. (G) IHC stains of 4-μm thick human tonsil FFPE tissue section for CD3 and CD20, which represent spatial distribution of T and B cells, respectively. SERS ratiometric images of human tonsil FFPE tissue stained with CD3-targeted BPE-flavored NPs (red), CD20-targeted 2NT-flavored NPs (white), and isotype control IgG-conjugated MPBA-flavored NPs. Scale bars for optical and SERS images represent 50 μm. Correlation of IHC and SERS NP staining was assessed through DAB optical density plotted as a function of SERS binding ratio for the FFPE tissue section. Linear trend with confidence band (95%) demonstrated correlation with r = 0.764 and r = 0.936 for T and B cells distribution relative to follicles, respectively. Error bars represent standard error of mean. Note: IHC stains were performed on two different tissue sections, while the Raman imaging channels were obtained from a single patient’s tissue specimen. Note: Raman images correlate well with the gold standard IHC stain, which demonstrated majority of T cells located outside the follicular zones and B cells observed primarily inside of follicles.
* DTTC (3,3’-diethyl-thiatricarbocyanine iodide), IR-775 chloride (2-[2-[2- chloro-3-[2-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene) ethylidene]-1-cyclohexen-1-yl]-ethenyl]-1,3,3-trimethyl-3H-indolium chloride), IR-780 iodide
(2-[2-[2-chloro-3-[(1,3-dihydro-3,3-dimethyl-1-propyl-2H-indol-2-ylidene)ethylidene]-1-cyclohexen-1-yl]ethenyl]-3,3-dimethyl-1-propylindolium iodide), IR-792 perchlorate (2-[2-[3-[(1,3-dihydro-3,3-dimethyl-1-propyl-2H-indol-2-ylidene)ethylidene]-2-(phenylthio)-1-cyclohexen-1-yl]ethenyl]-3,3-dimethyl-1-propylindolium perchlorate), IR-797 chloride (2-[2-[2-chloro-3-[2-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)ethylidene]-1-cyclopenten-1-yl]-ethenyl]-1,3,3-trimethyl-3H-indolium chloride), BMMBP (4,4’-bis(mercaptomethyl)biphenyl), 44DP (4,4’-dipyridyl), BPE (1,2-bis(4-pyridyl)ethylene), PODT (5-(4-pyridyl)-1,3,4-oxadiazole-2-thiol), PTT (5-(4-pyridyl)-1H-1,2,4-triazole-3-thiol), SERS (surface-enhanced Raman scattering), SERRS (surface-enhanced resonance Raman scattering), DFT (density functional theory), NP (nanoparticle).
Author
University of Southern California