Background: Clinical outcomes of hematopoietic stem cell transplantation (HSCT) following host hematopoiesis irradiation are variable, and early prediction of transplantation outcomes is crucial to manage relapses effectively [1]. 18F-fluorothymidine (18F-FLT) positron emission tomography (PET) is promising in this regard [2-5], yet manual segmentation and analysis of different bone segments in 18F-FLT PET images is time-consuming and hinders its clinical adoption.
Methods: This study explores the use of a deep neural network (3D U-Net) to facilitate segment-based analysis [6,7](Fig.1A), aiming to identify predictive imaging features for relapses post-HSCT. A retrospective study included 22 patients (18–50 years) who received 18F-FLT PET scans within 12 h before (d0), 5-12 days after (d5-12), and 28 days after irradiation (d28), with follow-up for up to one year to monitor relapses. The dataset contains 45 CT volumes and ten manually segmented CT volumes were used to train the 3D U-Net, which was tested on the remaining 35 volumes. A random-forest model was employed to analyze 18F-FLT uptake data and identify segments with the high predictive value of relapsing events based on feature importance score using the inherent explainer of scikit-learn. A threshold by which to predict relapsing events based on segments was found using the Youden Index.
Results: The 3D-U-Net allowed accurate segmentation of bones based on CT volumes (Fig. 1B&C), with a high accuracy illustrated by the confusion matrix (Fig.1D) and an average dice score of 0.965 in the testing dataset. Segment-based analysis clearly identified differences in 18F-FLT uptake between relapsing and non-relapsing patients at d0 and d5-12 in different segments, particularly in thoracic and lumbar/sacrum regions. Notable higher intensities at d0 in relapsing patients compared to non-relapsing patients were observed (Supporting Fig.1A-E). The random-forest model identified 18F-FLT uptake in thoracic segment at d0 as a significant predictor of relapse (Fig.1E), which was consistent with the observation of remnant 18F-FLT signal at thoracic region in images (Fig.1F). To facilitate clinical adoption, a threshold of 0.14% ID/g of the thoracic region at d0 was developed, which was shown to effectively distinguish relapsing from non-relapsing patients in our cohort (p<0.0053). In contrast, the pelvis region, which conventionally was thought of as an important predictor, did not show significant predictive value (Fig.1G).
Conclusions: This study demonstrates the clinical utility of 3D segmentation by deep neural networks for monitoring HSCT patients, revealing the thoracic region at d0 as a critical imaging feature predictive of relapse. The threshold developed in this study could also be easily used to facilitate the decision-making process of clinicians in managing patients receiving HSCT treatment.
Image/Figure: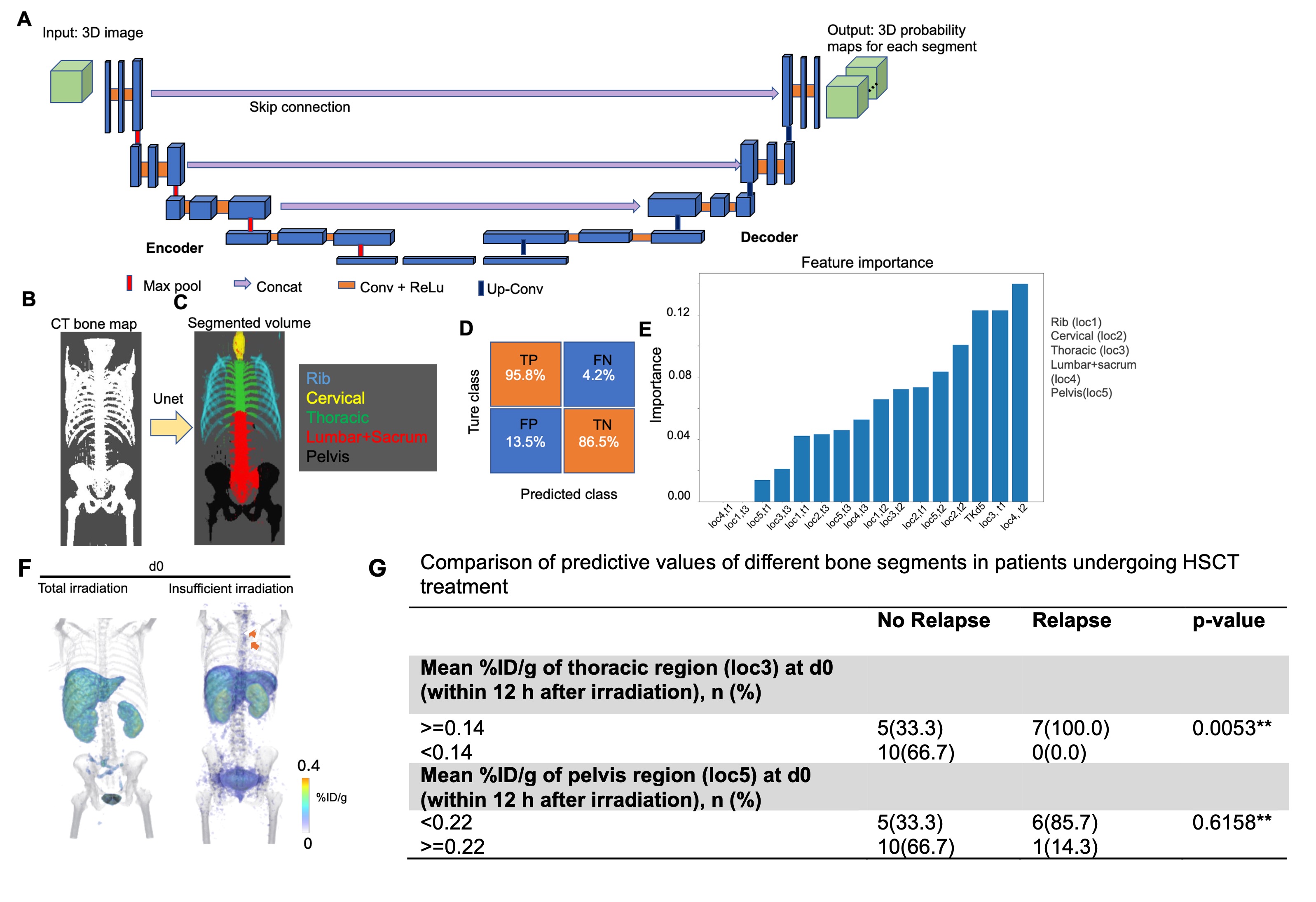
Click to view full size
Image/Figure Caption:
Fig.1. A. Illustration of the architecture of 3D-Unet architecture, which consists of a four-layer encoder and a four-layer decoder, which contracts and expands dimensions of 3D CT volumes in a step-wise manner. (B, C) An exemplary image showing color-coded segmented volume (C) based on the corresponding CT bone map (B). D. A confusion matrix showing high prediction performance of the trained Unet. E. Feature importance values of different segments in determining relapsing risk as unveiled after training a random forest model. F. Representative 3D rendering of whole-body PET volumes (jet color) co-registered with CT volumes (gray) within 12 after irradiation. A patient showing total bone marrow irradiation (left) and another patient with insufficient irradiation (right) are shown. Arrow: remnant 18F-FLT uptake in thoracic region. G. Value of segment-based analysis of thoracic region and pelvis region in predicting relapses at d0 (within 12 h after irradiation).
Author
Assistant Professor
University of Central Oklahoma