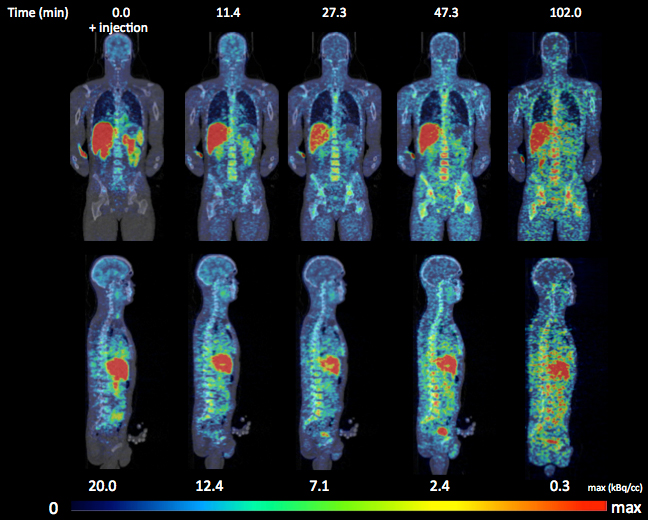
Biomarkers of (neuro)inflammation are useful as a diagnostic tool in drug development and therapy follow-up. The type 2 cannabinoid receptor (CB2R) is predominantly expressed in peripheral tissues and shows the highest expression levels in organs of the immune system [1]. In the central nervous system, CB2R is upregulated in inflammatory conditions of activated microglia. There has been growing interest in developing radioligands for noninvasive PET imaging of the CB2R. Most of the radioligands described in the literature are derivatives of 2-oxoquinoline class that have shown a high selectivity for CB2Rs as inverse agonists [2-6]. Although these radioligands were promising in in vitro studies, one of the problems in vivo was that they were unstable, resulting in fast metabolisation. On the other hand Horti et al. provided proof of principle for CB2R imaging in pathological conditions with a neuroinflammatory component [7]. Recently we have developed and validated novel specific radioligand for CB2R PET 2-oxo-7-[11C]-methoxy-8butyloxy-1,2-dihydroquinoline-3-carboxylic acid cyclohexylamide ([11C]-NE40), with favourable characteristics for neuroimaging. In the present study we wanted to evaluate the safety and usability of [11C]-NE40 in humans. Six healthy male subjects received a series WB dynamic scans after injection of [11C]-NE40. The dosimetry study on [11C]-NE40 resulted in an ED estimate of 3.64 µSv/MBq. The subjects in this study received a total ED of 1.06 ± 0.35 mSv for the PET and 1.0 mSv for the CT parts. In a recent MEDLINE literature search study with 32 human PET radiation dosimetry of carbon-11 radioligands, van der Aart et al. showed that all but one carbon-11 PET tracer have an effective dose under 9 microSv/MBq, with a mean of 5.9 microSv/MBq [6]. The effective radiation dose in our study is thus substantially less than the estimated maximum radiation burden caused by carbon-11 tracers. This means that [11C]-NE40 can be used in multiple PET scans in the same subject within conventionally accepted dose limits, facilitating its use in drug development and therapy follow-up.
The distribution pattern of [11C]-NE40 shows predominant hepatobiliary excretion pattern and is similar to the preclinical study [8]. The gallbladder shows a large variability in activity due to large differences in the individual kinetics of gallbladder emptying, a process that is influenced by multiple hormonal factors and gastrointestinal interaction. Rapid uptake and washout in the brain was seen, in accordance with the low CB2R expression levels in normal brain. The average injected tracer mass dose in this study was less than 1.0 µgram of NE40 and did not produce any subjective or clinically meaningful changes in laboratory blood tests, blood pressure, pulse and ECG. No adverse events were reported in this study.
Conclusion
[11C]-NE40 results in an effective human radiation dose of 3.64 µSv/MBq, which is the conventional range of 11C- tracers. A 300 MBq injection of [11C]-NE40, which will be used in the kinetic modelling studies, would result in a radiation dose of 1.1 mSv. The biodistribution with spleen uptake and absence of fixed brain uptake in healthy conditions makes the tracer promising for further studies in pathological conditions.
Ahmad R, Koole M, Evens N, Serdons K, Verbruggen A, Bormans G, Van Laere K.
Division of Nuclear Medicine, University Hospital Leuven, Herestraat 49, 3000, Leuven, Belgium. rawaha.ahmad@uzleuven.be
Source: Rawaha Ahmad
References
- Raitio KH, Savinainen JR, Nevalainen T, Jarvinen T, Vepsalainen J (2006) Synthesis and in vitro evaluation of novel 2-oxo-1,2-dihydroquinoline CB2 receptor inverse agonists. ChemBiolDrug Des 68:334-340.
- Gao M, Wang M, Miller KD, Hutchins GD, Zheng QH Synthesis and in vitro biological evaluation of carbon-11-labeled quinoline derivatives as new candidate PET radioligands for cannabinoid CB2 receptor imaging. BioorgMedChem 18:2099-2106.
- Evens N, Bosier B, Lavey BJ, et al. (2008) Labelling and biological evaluation of [(11)C]methoxy-Sch225336: a radioligand for the cannabinoid-type 2 receptor. Nucl Med Biol 35:793-800.
- Evens N, Muccioli GG, Houbrechts N, et al. (2009) Synthesis and biological evaluation of carbon-11- and fluorine-18-labeled 2-oxoquinoline derivatives for type 2 cannabinoid receptor positron emission tomography imaging. Nucl Med Biol 36:455-465.
- Turkman N, Shavrin A, Paolillo V, et al. Synthesis and preliminary evaluation of [18F]-labeled 2-oxoquinoline derivatives for PET imaging of cannabinoid CB2 receptor. NuclMedBiol 39:593-600.
- van der Aart J, Hallett WA, Rabiner EA, Passchier J, Comley RA Radiation dose estimates for carbon-11-labelled PET tracers. NuclMedBiol 39:305-314.
- Horti AG, Gao Y, Ravert HT, et al. Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2). BioorgMedChem 18:5202-5207.
- Evens N, Vandeputte C, Coolen C, et al. (2012) Preclinical evaluation of [11C]NE40, a type 2 cannabinoid receptor PET tracer. NuclMedBiol 39:389-399.
